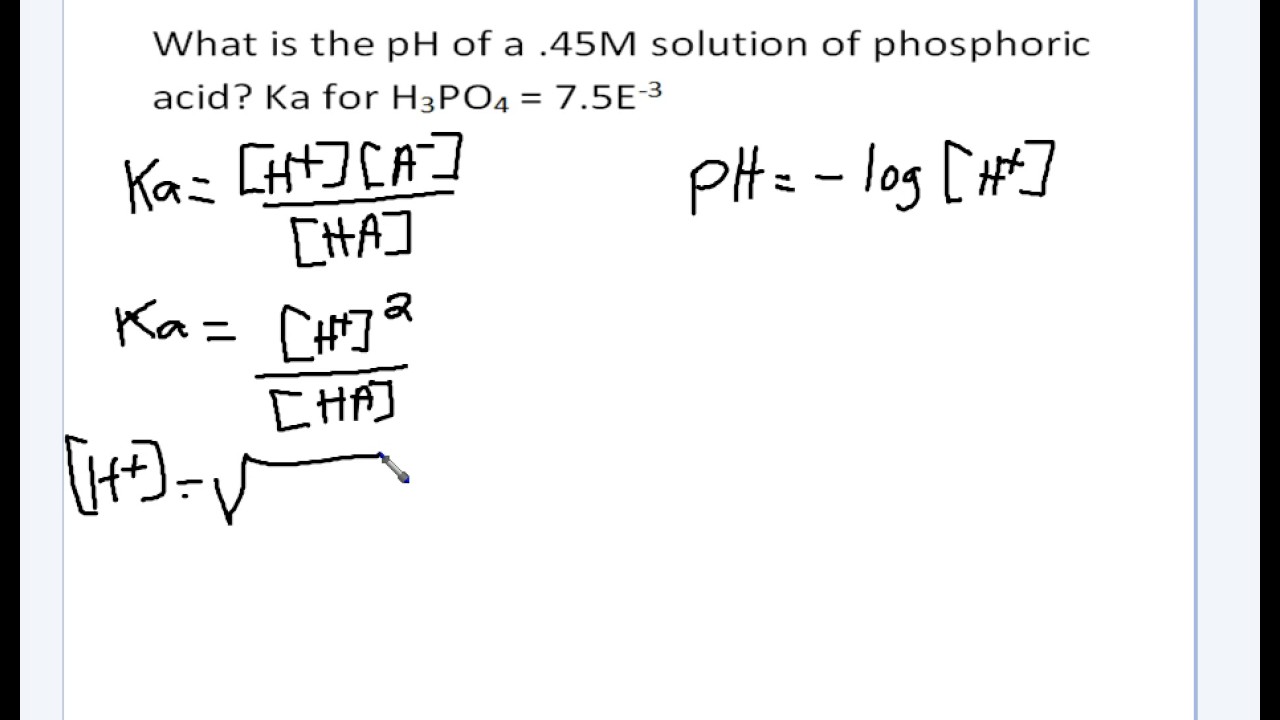
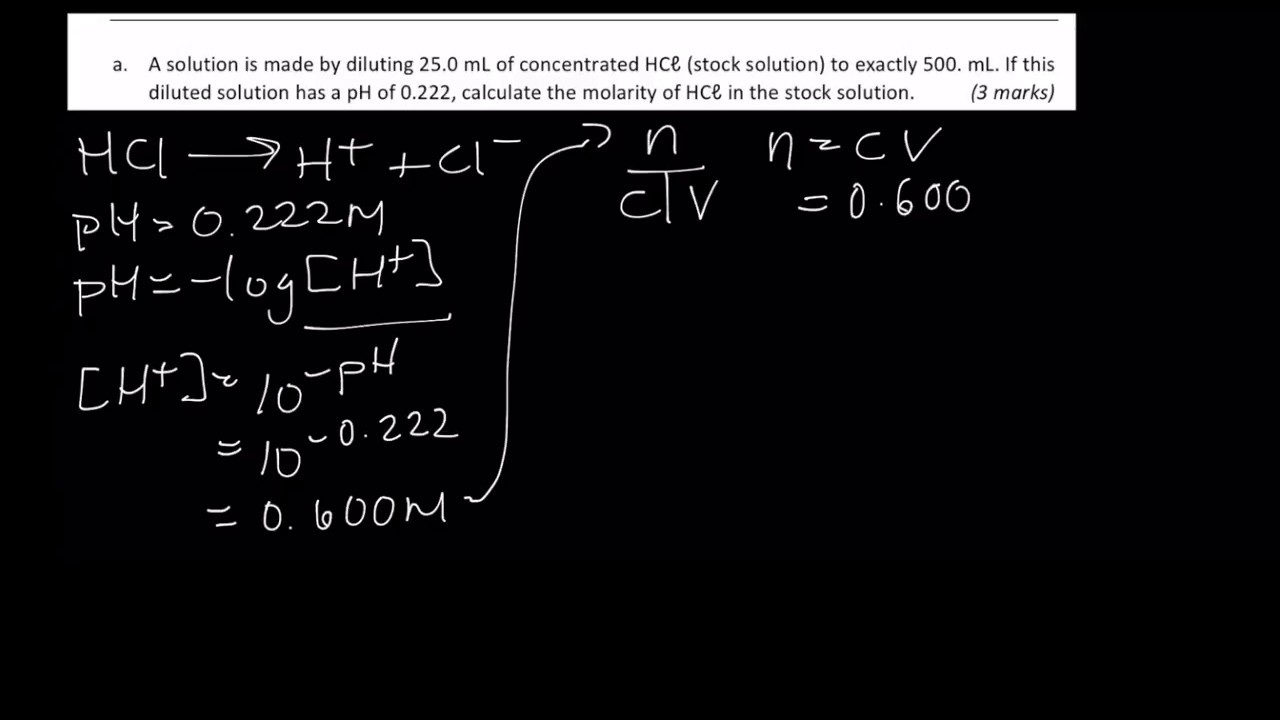
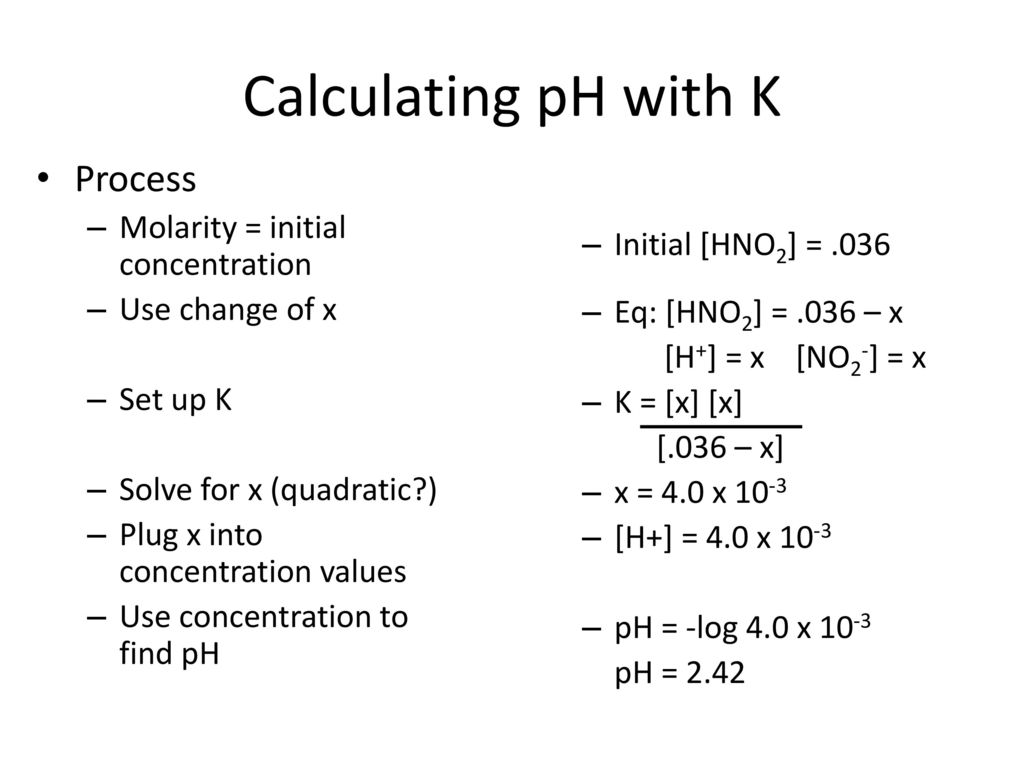
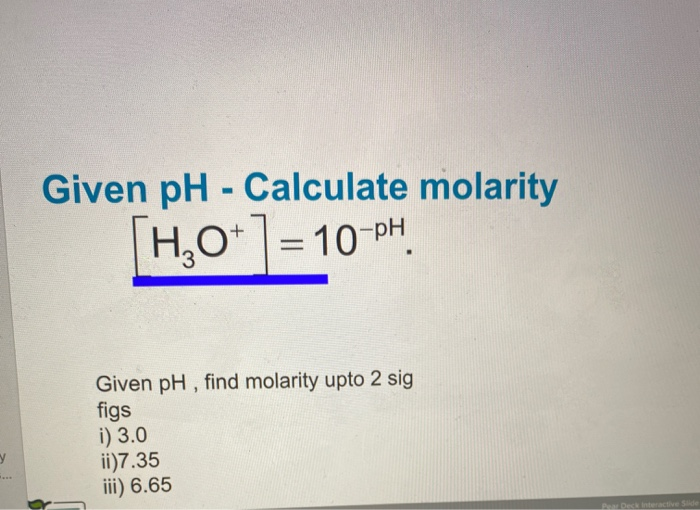
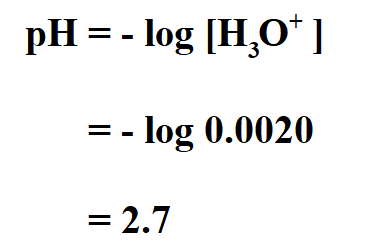![pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarity… | Chemistry lessons, Teaching chemistry, Chemistry education pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarity… | Chemistry lessons, Teaching chemistry, Chemistry education](https://i.pinimg.com/originals/99/80/05/998005d7b3fbb74f7a91222f3209e7c5.png)
pH = -log[H+] assuming 100 percent dissociation; if given percent ionization, multiply by the molarity… | Chemistry lessons, Teaching chemistry, Chemistry education
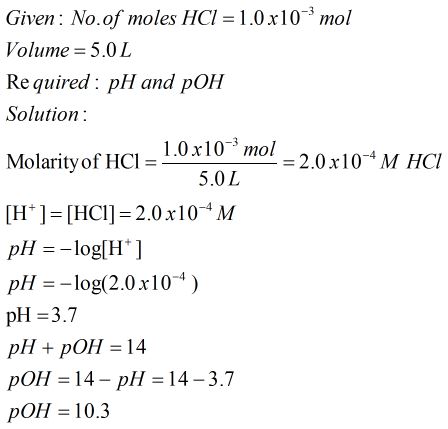
![SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice](https://cdn.numerade.com/ask_previews/f6745ba3-6b77-4e7a-9de2-7d980958d194_large.jpg)
SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice
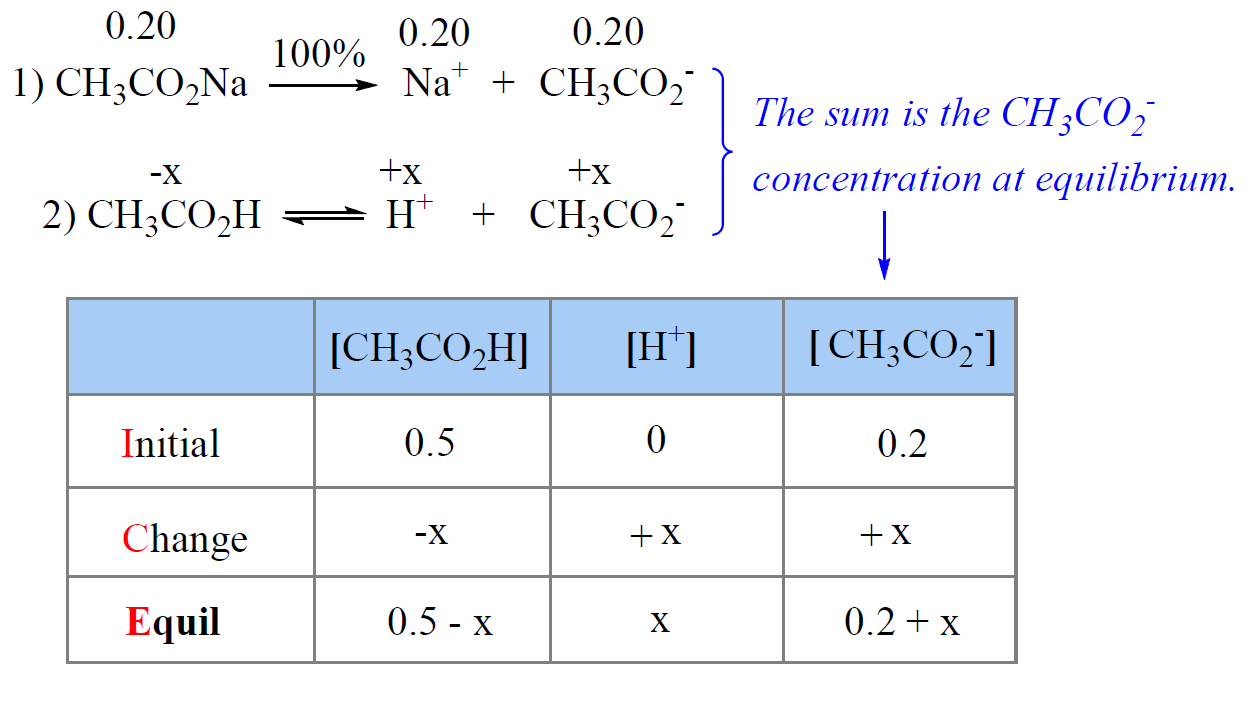
![SOLVED: 1. What is the pH of stomach acid, a solution of HCI with a hydronium ion concentration of 1.2 x 10-3M? 2. A solution has a OH- concentration [OH-] = 4.5 SOLVED: 1. What is the pH of stomach acid, a solution of HCI with a hydronium ion concentration of 1.2 x 10-3M? 2. A solution has a OH- concentration [OH-] = 4.5](https://cdn.numerade.com/ask_images/9803e13770894e00af95303eb4bf8bbc.jpg)
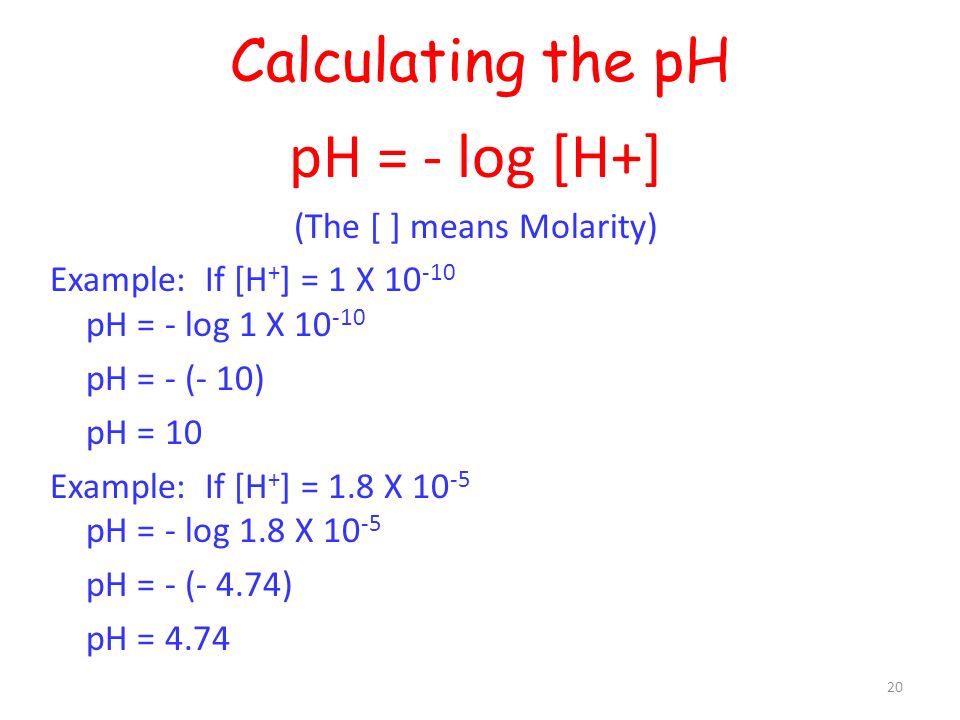
![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)