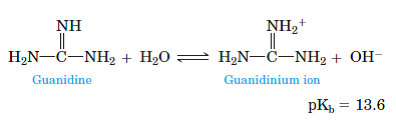Guanidine and the guanidino group present in arginine are two of the strongest organic bases known. Account for their basicity. | Homework.Study.com

Sequential Reduction of Nitroalkanes Mediated by CS2 and Amidine/Guanidine Bases: A Controllable Nef Reaction | Organic Letters

Figure 1 from Very strong organosuperbases formed by combining imidazole and guanidine bases: synthesis, structure, and basicity. | Semantic Scholar
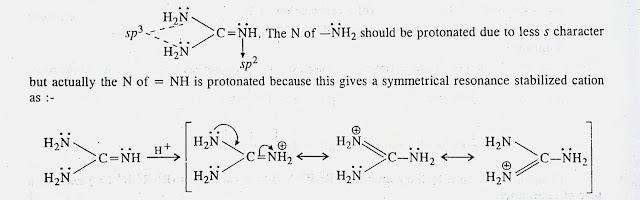
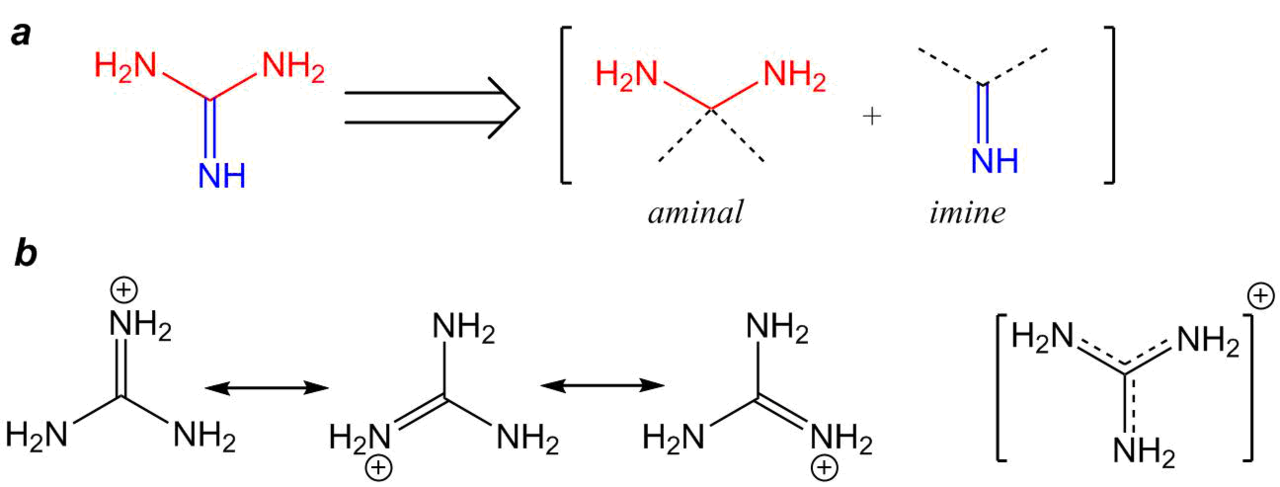
Welcome to Chem Zipper.com......: Give an explanation for the fact that Guanidine NH=C(CH3)2 is a stronger base than most of amines?
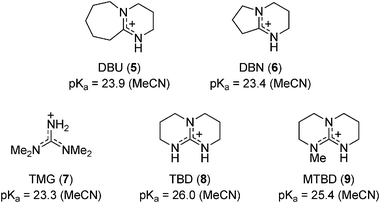
Amidines , isothioureas, and guanidines as nucleophilic catalysts - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C2CS15288F

C2‐Symmetric Chiral Pentacyclic Guanidine: A Phase‐Transfer Catalyst for the Asymmetric Alkylation of tert‐Butyl Glycinate Schiff Base - Kita - 2002 - Angewandte Chemie - Wiley Online Library
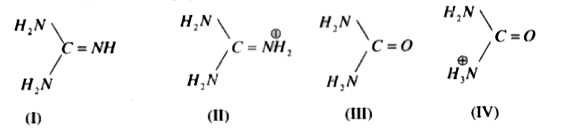
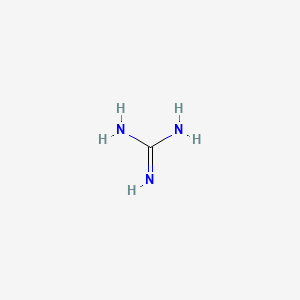
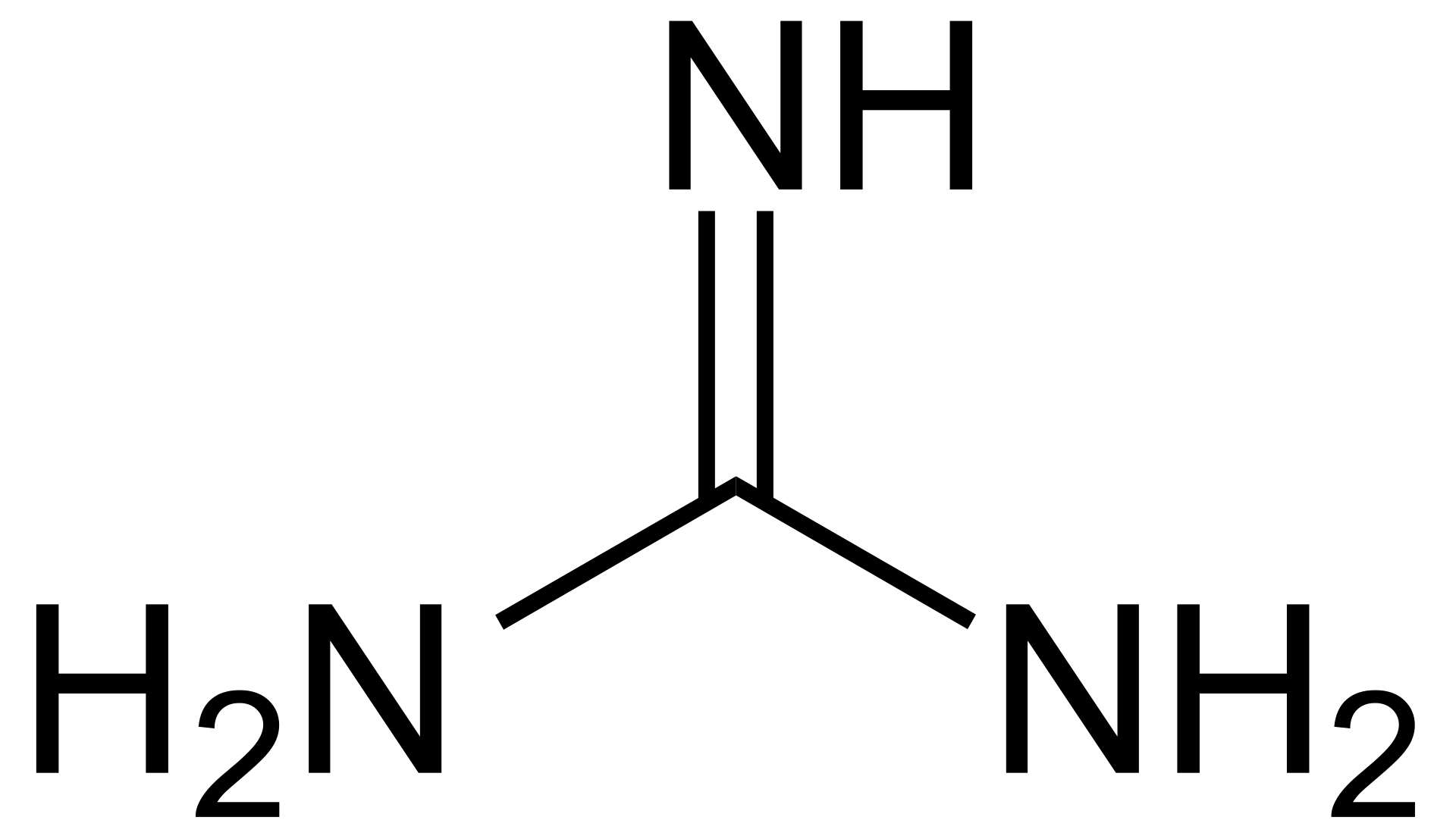
Guanidine (I) and its conjugate acid (II) are given below along with urea(III) and its conjugate base (IV) Basic properties of I & II compounds are mainly influenced by resonance and the

Strong Bicyclic Guanidine Base-Promoted Wittig and Horner−Wadsworth−Emmons Reactions | Organic Letters
SOLVED: Guanidine is the strongest base among neutral organic compounds. The reason for the greater basicity of guanidine is: A. presence of three nitrogen atoms in the compound B. The delocalization of

Superbases based on guanidine and the values of pKa of the conjugated... | Download Scientific Diagram
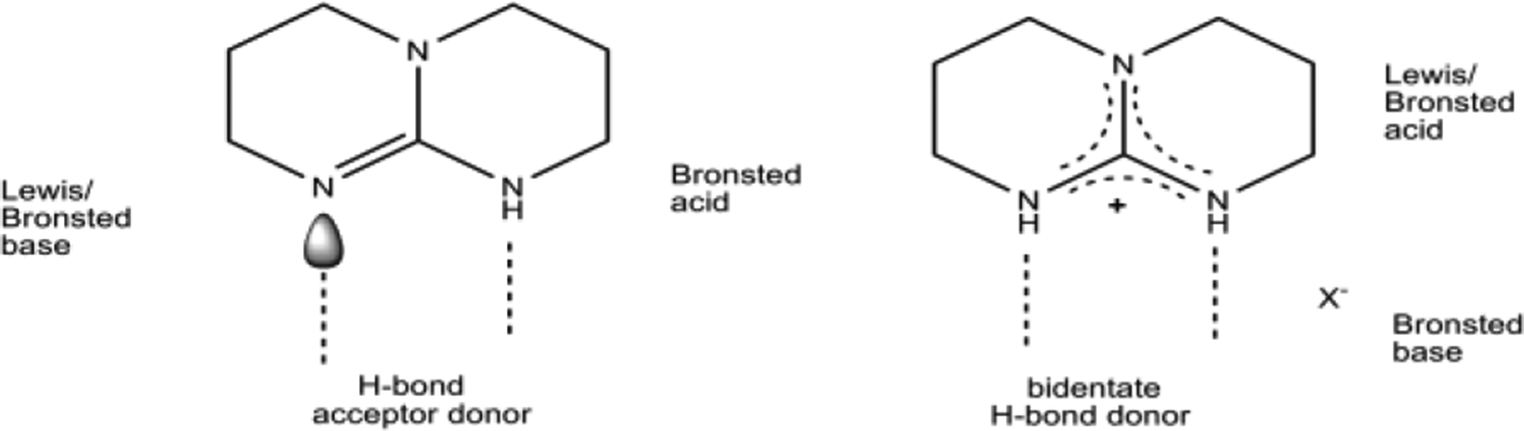
Recent Advances in Guanidine-Based Organocatalysts in Stereoselective Organic Transformation Reactions | IntechOpen