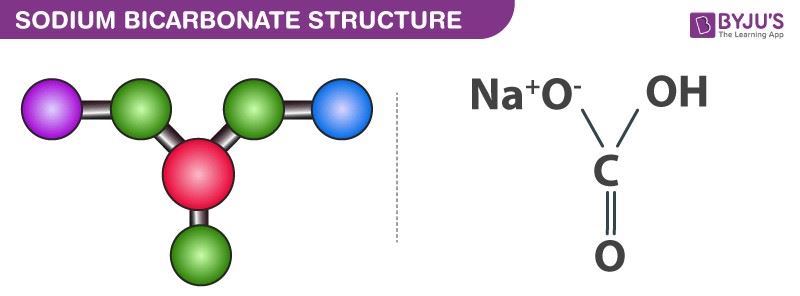
![SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 . SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 .](https://cdn.numerade.com/previews/bd62afea-5060-4429-b9f4-4515705d9462_large.jpg)
SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 .
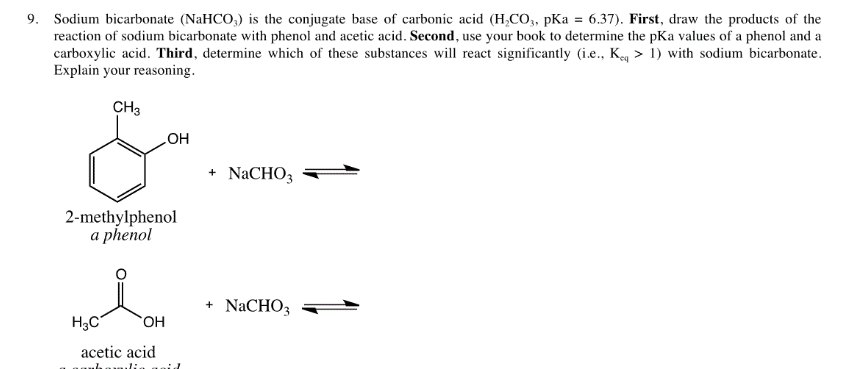
✓ Solved: In Experiment 3, the benzoic acid could have been extracted from the ether layer using sodium...

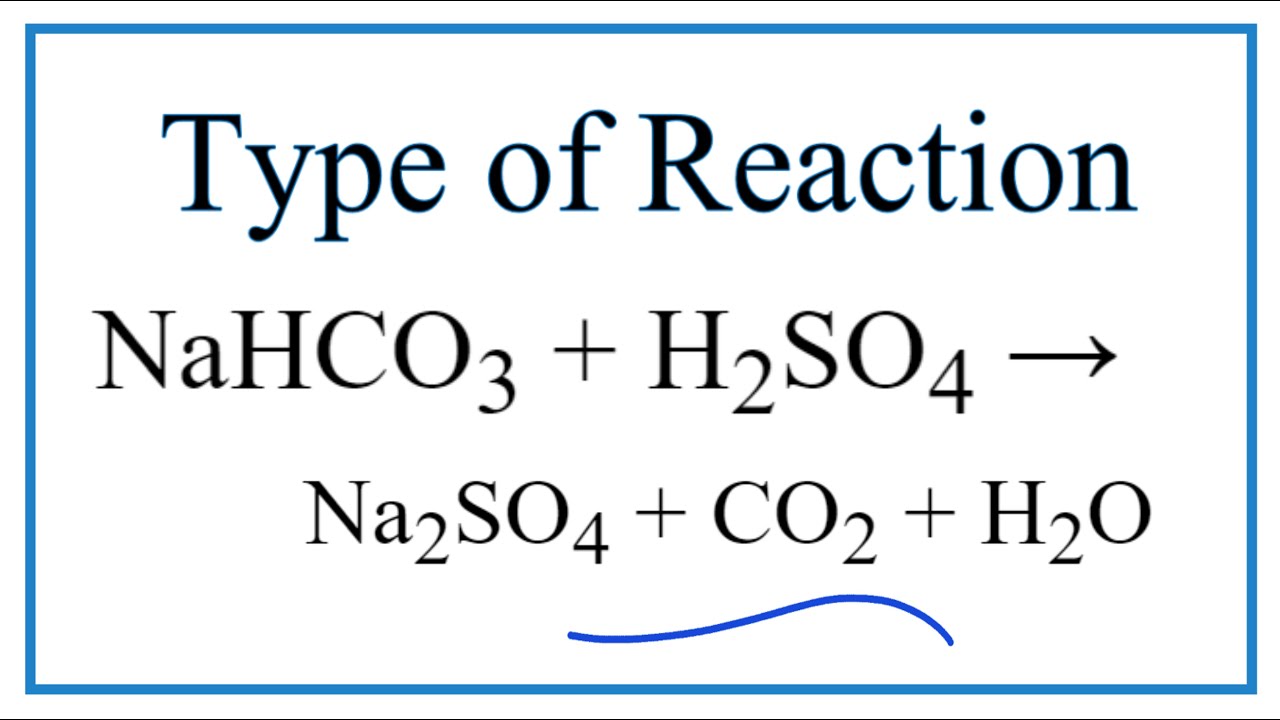
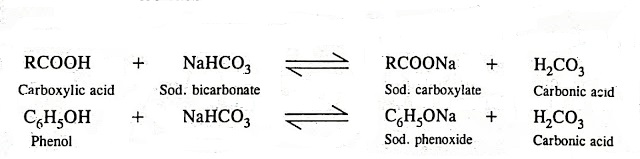
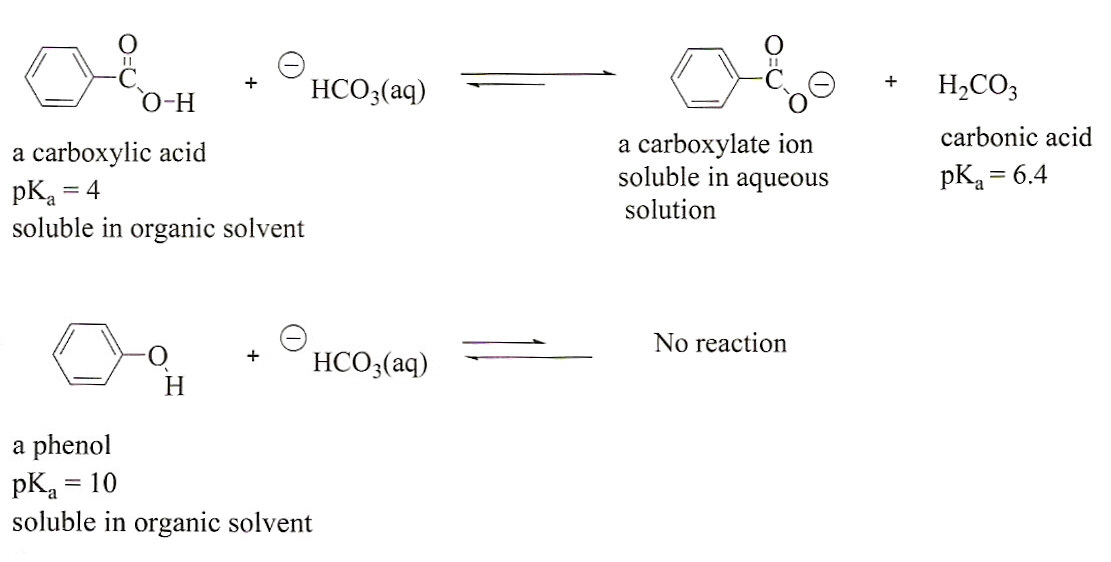
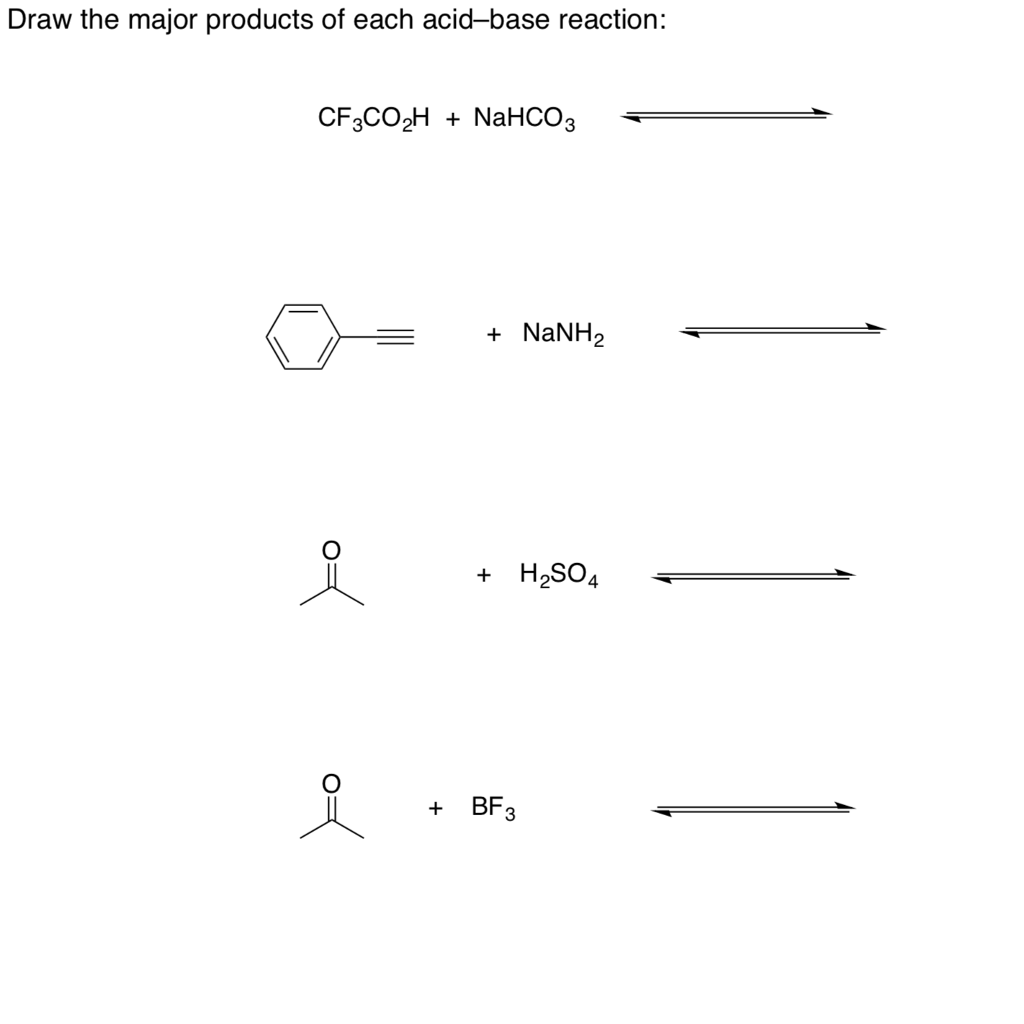
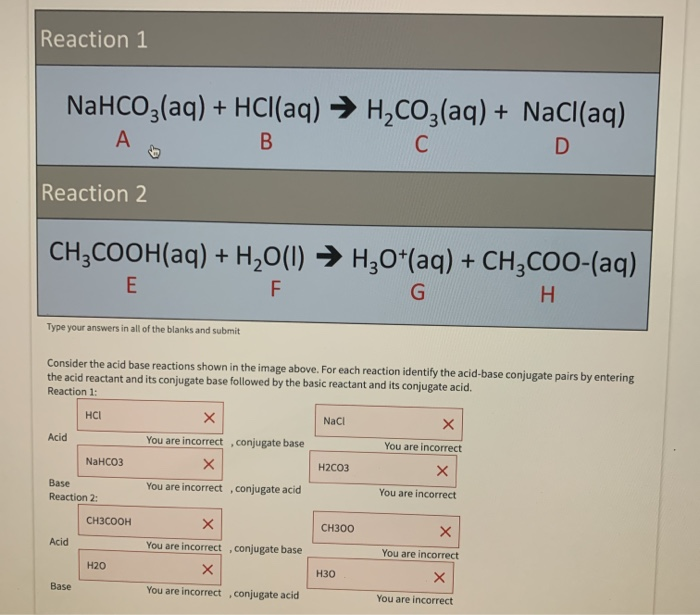
NaHCO3 + CH3COOH⟶ CH3COONa + CO2 + H2O ,This reaction is used as a test of carboxylic acid because :

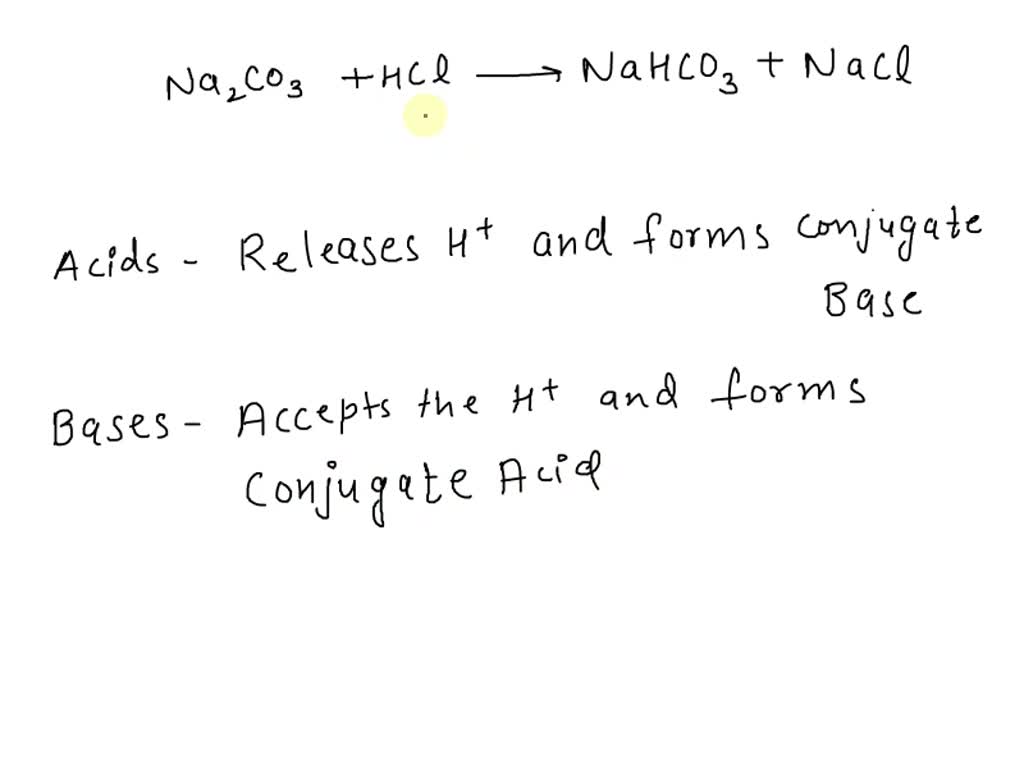
Acid–base titration curves of the biomass pretreated with NaHCO3 sat.... | Download Scientific Diagram




![ANSWERED] Acetic acid (CH3COOH) is the acid in vine... - Physical Chemistry ANSWERED] Acetic acid (CH3COOH) is the acid in vine... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/60677547-1657054377.0366533.jpeg)